A 0.100
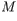
solution of ethylamine (

) has a

of 11.87. Calculate the

for ethylamine
So I took the pH and calculated that x = 7.4 x 10^-3 M
THen I set up one of those ICE charts:

+ H20 --> C2H5NH3+ + OH-
Initial 0.100 ---- ----
Change -x M + x M + x M
EQuil. Concen 0.100 - x M x M x M
Then I used Kb = x^2 divided by (0.100- x)
My answer was 5.9 x 10^-3 but that is not the right answer. I don't know where I went wrong
 Free Forum Hosting
Free Forum Hosting