 |
 |
Reply
 | | From:   alexbigal2 (Original Message) alexbigal2 (Original Message) | Sent: 10/21/2008 1:09 AM |
Meltable, Moldable Nitrate Explosive</TI> 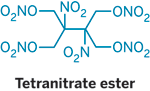 Chemists have synthesized a novel nitrate ester explosive that has the rare and desirable property of being a solid with a melting point low enough so that it can be poured into molds for casting into different shapes (Angew. Chem. Int. Ed. 2008, 47, 8306). David E. Chavez and colleagues at Los Alamos National Laboratory report that the compound, which sports four nitrate ester groups (ONO2) and two nitro groups (NO2), melts at 85 ºC, well before its decomposition point of 141 ºC. The nitrate ester family of explosives got started with the discovery of nitroglycerin in 1846. However, almost all of the compounds are liquids at ambient temperatures. Nitrocellulose is one example of a solid nitrate ester, as is pentaerythritol tetranitrate (PETN). But PETN has a high melting point, requiring that it be pressed into desired shapes. The new tetranitrate ester shares PETN's sensitivity to friction and sparks, and it has explosive capabilities like those of the compound HMX, a cyclic tetranitro compound that is one of the highest performing explosives. |
|
 First First
 Previous
2-3 of 3
Next Previous
2-3 of 3
Next Last
Last
|
Reply
 | | | From: Jaom | Sent: 10/23/2008 2:38 PM |
Isnt it quite dangerous to synthesize such things, the lab work...and is it really necessary?! |
|
Reply
 | | From:   ·Steve· ·Steve· | Sent: 10/23/2008 9:41 PM |
Agreed. This compound is abundantly referenced in the online science magazines, and as such, it is well-known. Not my cup of tea, that's for sure. But explosives, in large amounts and in tiny amounts as well, are used extensively in a wide variety of applications. The World of Explosives |
|
|
 Free Forum Hosting
Free Forum Hosting