I'll take these one at a time!
1. If there is not enough acid present, the following reaction can occur:
[Fe(H2O)n]2+ (aq) 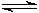 [Fe(H2O)n�?(OH)]+ (aq) + H+ (aq)
[Fe(H2O)n�?(OH)]+ (aq) + H+ (aq)
This will be minor since the Fe2+ ions are complexed by the o-phen ligands, but it is a possibility. Adding acid ensures that the equilibrium will favor the product (Le Chatelier) so there will not be any hydroxo complex present. If the pH is high enough, solid particles of Fe(OH)2 would form and precipitate out, adversely affecting the result.
Steve
Hello,
I have a couple of questions regarding Beer's Law.
1) In order to prevent hydrolysis reaction of Fe2+ solution with water, acid must be added to the standard iron
solution. Student forgot to add acid to the standard solution. What change this will have on student final
results?
I think because the acid was not added then the less iron(II) ions present in the sample so more light will be transmitted through.
|
 Free Forum Hosting
Free Forum Hosting