Both of these problems involve two parts:
1. Calculate the amounts of everything in the reaction after adding the HCl or NaOH, and
2. Calculate the pH
The first problem is the easiest. Here is the reaction and the initial amounts present and the amounts after the reaction:
NH3 (aq) + HCl (aq) ––�?gt; NH4Cl (aq)
Initially: 0.08 mol 0.06 mol 0
After Rxn: 0.02 mol 0 0.06 mol
HCl is the limiting reactant and NH3 is in excess.
To calculate the pH, you can then simply plug into the Henderson-Hasselbalch equation:
pH = pKa + log (moles of NH3 / moles of NH4+)
Note that you can use moles in the last term and do not have to convert to molarity, because the total volume of the solution cancels when you divide moles in the numerator and denominator by the total volume to get molarities.
The second problem is actually more complicated, because NaOH is not a strong enough base to react completely with phosphoric acid in aqueous solution. The first two acidic hydrogens in H3PO4 do react completely, but the third hydrogen does not react completely and you end up with an equilibrium mixture:
H3PO4 (aq) + 2 NaOH (aq) ––�?gt; Na2HPO4 (aq) + 2 H2O (l)
Initially: 0.015 mol 0.08 mol 0 �?BR>After Rxn: 0 0.05 mol 0.015 mol �?/FONT>
Na2HPO4 (aq) + NaOH (aq) 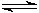 Na3PO4 (aq) + H2O (l)
Na3PO4 (aq) + H2O (l)
Initially: 0.015 mol 0.05 mol 0 �?BR>At Equilibrium: 0.015 �?x 0.05 �?x x �?/FONT>
If we can calculate x, we can calculate the moles of NaOH and thus can obtain the OH�?/SUP> concentration, from which we can calculate the pH. To do so, we need to know the value of the equilibrium constant for this reaction. I can calculate a value by adding the following two reactions together (I'll omit the spectator Na+ ions for simplicity):
HPO42�?/SUP> (aq) 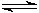 H+ (aq) + PO43�?/SUP> (aq) K3 = 4.8 X 10�?3
H+ (aq) + PO43�?/SUP> (aq) K3 = 4.8 X 10�?3
H+ (aq) + OH�?/SUP> (aq) 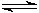 H2O (l) K = 1/Kw = 1.0 X 1014
H2O (l) K = 1/Kw = 1.0 X 1014
__________________________________________________________________________
HPO42�?/SUP> (aq) + OH�?/SUP> (aq) 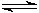 PO43�?/SUP> (aq) + H2O (l) Keq = 48
PO43�?/SUP> (aq) + H2O (l) Keq = 48 I once found a website that gave the H3PO4, NaH2PO4, Na2HPO4, and Na3PO4 concentrations when various amounts of NaOH were present, but I did not run across it or a similar one this time. The message search here did not find it either (that was several years ago). But as I recall, the Keq value I derived from the website data was somewhat different from the value of 48 I obtained above. 
It is more probable that you are supposed to assume that the H3PO4 reacts completely (most people do not realize that it does not, unless a very large excess of NaOH is present). This would give,
H3PO4 (aq) + 3 NaOH (aq) ––�?gt; Na3PO4 (aq) + 3 H2O (l)
Initially: 0.015 mol 0.08 mol 0 �?BR>After Rxn: 0 0.035 mol 0.015 mol �?/FONT>
In this case, you can approximate the pH of the solution based on the concentration of hydroxide ion from the NaOH that remains in excess and ignore the effect of the Na3PO4. This may be what is expected from the problem.
Steve
 Free Forum Hosting
Free Forum Hosting