Hi Nayi, first, to find out which way the reaction will go initially in order to reach equilibrium, you need to calcualte the reaction quotient, Q, by plugging the initial values into the K expression, and then compare the value of Q to K. (I'll label these Qp and Kp since we are using pressure units).
Kp = [H2O]2 [Cl2]2 = 1.96 X 109
[HCl]4 [O2]
Qp = (0.1 atm)2 (0.2 atm)2 = 0.064
(0.5 atm)4 (0.1 atm)
Since Qp < Kp, the reaction will go in the forward (-->) direction in order to reach equilibrium.
Or,
4HCl + O2 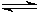 2H2O + 2Cl2
2H2O + 2Cl2
initially 0.5 atm 0.1 atm 0.1 atm 0.2 atm
change -4x -x +2x +2x
at equlibrium 0.5 - 4x 0.1 - x 0.1 + 2x 0.2 + 2x
If you plug the equlibrium terms into the Kp expression to solve for x, you will have a complicated 5th order polynomial to find the roots of, unless it can be simplified. What they normally do in these cases is to provide one more bit of information, such as the equilibrium concentration of one substance, so you can calculate the value of x immediately. With such a large equilibrium constant, we know the reaction heavily favors the products at equilibrium so I'm not surprised that x is about 0.1, the initial partial pressure of the limiting reactant, O2. It should actually be a little less, so you will not have a zero in the denominator. Doing a little trial and error, it looks like x must be between 0.099999926 and 0.099999927. Here is the expression I was using:
(0.1 + 2x)2 (0.2 + 2x)2 = 1.96 X 109
(0.5 - 4x)4 (0.1 - x)
Perhaps they simply approximated that x = 0.1 atm based on the very large value of Kp.
Steve
 Free Forum Hosting
Free Forum Hosting