Small molecules selectively targeting four-stranded DNA could lead to anticancer drugs without the toxic side-effects, say scientists in the UK.
"The small molecules aren't toxic to healthy cells, and could specifically knock out cancer cell growth."
- Stephen Neidle
Quadruplex DNA is a four-strand form of the biomolecule and is associated with cancer cell replication. Stephen Neidle and William Drewe at the University of London designed a series of diphenyl urea-based structures that bind strongly to quadruplex DNA but have low affinity for duplex DNA - the two strand double helix. This selectivity means the molecules aren't toxic to healthy cells, and could specifically knock out cancer cell growth, says Neidle.
Guanine-rich DNA quadruplexes (G4s) can be found in many telomeres, end sections of chromosomes which in healthy tissue gradually degrade until the cell can no longer divide. But in cancer cells, telomeres are continually maintained by telomerase - an enzyme switched off in most healthy adult tissue - effectively making cancer cells immortal. Neidle and Drewe's molecules are designed to disrupt telomerase from binding to the quadruplex.
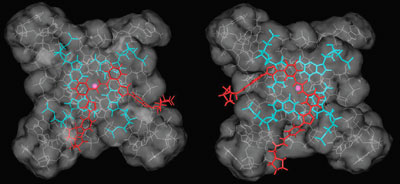
| Molecular modelling studies suggested that diphenyl ureas would bind to guanine-rich DNA quadruplexes |
'The defining characteristic of most drugs is that they are toxic to most cells, including healthy ones,' says Neidle. 'We're looking for molecules that don't kill cells, but have a telomerase blocking mechanism.' While a number of molecules have been found to bind G4 DNA, these molecules have typically been unselective, and too large to be useful as drug molecules. 'The idea was to move away from polycyclics and develop a much more drug-like framework,' he adds.
Neidle and Drewe used click chemistry to link a biaryl urea core to two azide side chains, which modelling studies had suggested would bind strongly to G4 DNA. This synthetic strategy uses reliable reactions to join small units together meaning that a variety of related compounds could be synthesised quickly for testing.
'Designing a drug-like quadruplex binder that really doesn't bind to duplex DNA is a pretty big step forward,' says Mark Searcy, who studies DNA quadruplex binding at the University of East Anglia, Norwich, UK. 'You're trying to target one DNA quadruplex among hundreds of thousands of duplexes, so you need very high selectivity,' he adds.
Having shown that the compounds selectively bind G4 DNA, and are not toxic to cells, Neidle is currently testing the compounds' ability to curb cancer cell growth.
James Mitchell Crow
 Free Forum Hosting
Free Forum Hosting