| 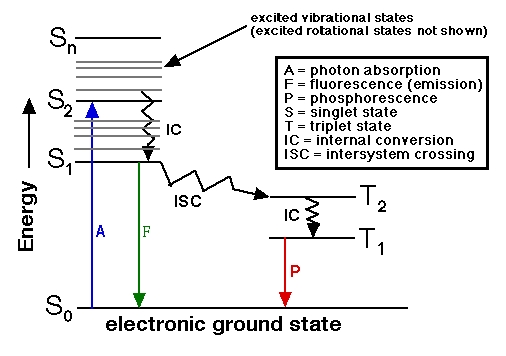 The terms "singlet" and "triplet" are spectroscopist's terms. In a magnetic field, a singlet molecule, or one that has all of its electrons paired in the same orbitals, ↑↓, or in a singlet excited state paired but in different orbitals, �? �?) exhibits only a single energy state, while a triplet molecule (a diradical having two unpaired electrons, �? �?/U> in different orbitals) exhibits three energy states corresponding to the possible orientations of the electron spins with respect to the magnetic field (both spins "up", both spins "down", or one spin "up" and the other "down"). A simple illustration using up and down arrows as electrons is shown at http://teaching.shu.ac.uk/hwb/chemistry/tutorials/molspec/lumin1.htm.
From the standpoint of total spin, if the total spin is zero (when all electrons are paired +½ and –�?adding up to zero) this is a singlet state. If you have two unpaired electrons in the molecule, +½ and +½, the total spin is +1. The spin multiplicity is given by 2S + 1. If the total spin is zero (S = 0) then the spin multiplicity 2S + 1 = 1 (a singlet state). If the total spin is 1 (S = 1) then 2S + 1 = 3 (the triplet state). Allowed electron spin transitions in atoms and molecules obey ΔS = ±1.
>> is it possible for electrons to go through ISC (Intersystem crossing) from Triplet state to Singlet state? <<
"Although intersystem crossing has been identified primarily with transitions from the lowest excited singlet state of a molecule to an even lower-lying triplet state, triplet to singlet intersystem crossing also may occur. Well-known examples of reverse (triplet to singlet) intersystem crossing include E- and P-type delayed fluorescence."
Steve
|
 Free Forum Hosting
Free Forum Hosting