|
|
 |
 |
Reply
 | | From:   goldie647 (Original Message) goldie647 (Original Message) | Sent: 4/13/2008 9:53 PM |
Hello,
I have a couple of questions regarding Beer's Law.
1) In order to prevent hydrolysis reaction of Fe2+ solution with water, acid must be added to the standard iron
solution. Student forgot to add acid to the standard solution. What change this will have on student final
results?
I think because the acid was not added then the less iron(II) ions present in the sample so more light will be transmitted through.
2). If 2.5 mL of a standard iron solution (1 mL = 0.050 mg Fe) is diluted to 100 mL, what is the final iron
concentration in mg Fe/mL?
Unsure of what the question is asking :(
4. A solution concentration 3.75 mg/ml of a sample 220 g/mol has a transmittance of 39.6% in a 1.50-cm cell at
480 nm. Calculate the molar absorptivity of sample.
I know I use Beers Law:
A=abc
solve for a = A/bc
I know A is equal to -log (39.6)
b, I am unsure of but I think it is 1.50 cm, I am confused on the wavelength value of 480nm.
c, I think should be 3.75 mg/ml, but what us the 220 g/mol for?
Thanks for any help
|
|
 First First
 Previous
2-10 of 10
Next Previous
2-10 of 10
Next Last
Last
|
Reply
 | |
Can I use %T= 100/10^(A)
to figure out the %T for the following:
For a solution of 0.200 M Cu (NO3)2 in a 1-cm cell, the following data has been collected. Calculate the
percentage transmittance, %T, for each absorbance.
C(mol/L) A % T
0.010 0.112
0.030 0.336
0.060 0.672
0.090 1.008
0.120 1.120
Thanks
|
|
Reply
 | |
Any help would be appreciated.
Thanks |
|
Reply
 | | From:   ·Steve· ·Steve· | Sent: 4/14/2008 6:05 PM |
I'll take these one at a time! 1. If there is not enough acid present, the following reaction can occur: [Fe(H2O)n]2+ (aq) 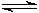 [Fe(H2O)n�?(OH)]+ (aq) + H+ (aq) [Fe(H2O)n�?(OH)]+ (aq) + H+ (aq) This will be minor since the Fe2+ ions are complexed by the o-phen ligands, but it is a possibility. Adding acid ensures that the equilibrium will favor the product (Le Chatelier) so there will not be any hydroxo complex present. If the pH is high enough, solid particles of Fe(OH)2 would form and precipitate out, adversely affecting the result. Steve Hello,
I have a couple of questions regarding Beer's Law.
1) In order to prevent hydrolysis reaction of Fe2+ solution with water, acid must be added to the standard iron
solution. Student forgot to add acid to the standard solution. What change this will have on student final
results?
I think because the acid was not added then the less iron(II) ions present in the sample so more light will be transmitted through.
| |
|
Reply
 | | From:   ·Steve· ·Steve· | Sent: 4/14/2008 6:11 PM |
2. For this one, just use the dilution formula V1C1 = V2C2 and solve for C2, the concentration of the diluted solution. V1 is 2.5 mL, C1 is 0.050 mg/mL, and V2 is 100 mL. Piece of cake!  2). If 2.5 mL of a standard iron solution (1 mL = 0.050 mg Fe) is diluted to 100 mL, what is the final iron concentration in mg Fe/mL?
Unsure of what the question is asking :(
|
|
Reply
 | | From:   ·Steve· ·Steve· | Sent: 4/14/2008 6:20 PM |
That is right, use a = A/bc. A = –log(T), not –log(%T), so A = –log(0.396). The path length is "b" in the equation, normally in cm units, so 1.50 cm is correct. You do not need the wavelength for the calculation, but since absorptivity depends on the wavelength, the wavelength is given as a formality. The concentration c can be in any units. However, since the question asks for the molar absorptivity, the concentration needs to be converted to moles per liter. That's why the molar mass was given, 220 g/mol. 4. A solution concentration 3.75 mg/ml of a sample 220 g/mol has a transmittance of 39.6% in a 1.50-cm cell at 480 nm. Calculate the molar absorptivity of sample.
I know I use Beers Law:
A=abc
solve for a = A/bc
I know A is equal to -log (39.6)
b, I am unsure of but I think it is 1.50 cm, I am confused on the wavelength value of 480nm.
c, I think should be 3.75 mg/ml, but what us the 220 g/mol for?
|
|
Reply
 | | From:   ·Steve· ·Steve· | Sent: 4/14/2008 6:25 PM |
>> Can I use %T= 100/10^(A) << Yes, that is correct.  Can I use %T= 100/10^(A)
to figure out the %T for the following:
For a solution of 0.200 M Cu (NO3)2 in a 1-cm cell, the following data has been collected. Calculate the percentage transmittance, %T, for each absorbance.
C(mol/L) A % T
0.010 0.112
0.030 0.336
0.060 0.672
0.090 1.008
0.120 1.120
|
|
Reply
 | | From:   ·Steve· ·Steve· | Sent: 4/15/2008 4:58 PM |
Q1. >> Adding acid ensures that the equilibrium will favor the product (Le Chatelier) << Ack! Adding acid ensures that the equilibrium will favor the reactant, [Fe(H2O)n]2+, since H+ ion is one of the products. I just noticed this error when I was checking for new messages. |
|
Reply
 | |
| Thanks so much Steve for all of your help. I am slowly getting it. :( |
|
 First First
 Previous
2-10 of 10
Next Previous
2-10 of 10
Next Last
Last
|
|
|
 Free Forum Hosting
Free Forum Hosting