2. I just plugged into the formula ΔG° = –RT lnKp :
ΔG° = �?8.314 X 10�? kJ mol�? K�?)(700 K) ln(5.10) = �?.48 kJ/mol.
CO (g) + H2O (g) 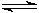 CO2 (g) + H2 (g)
CO2 (g) + H2 (g)
Keq = [CO2][H2]
[CO][H2O]
Since the concentrations of ideal gases are proportional to their pressures, we can say,
Kp = PCO2 PH2
PCO PH2O
which is used in the equation ΔG° = –RT ln Kp
 Free Forum Hosting
Free Forum Hosting