To answer these, it is necessary to remember a few trends. For ionic compounds, we take each ion separately and identify it as being acidic, basic, or neutral. Then we can usually say what the net effect of both of the ions in the compound will be.
NaCl
1) Na+ ion
The group IA ions do not affect the pH to any significant degree, so Na+ ion is neutral.
2) Cl- ion
The negative ion (that is, the conjugate base) of a strong monoprotic acid like HCl is such a weak base that it is neutral. The general trend is, the stronger the acid, the weaker its conjugate base. For strong acids like HCl, HBr, HI, HNO3, or HClO4, for instance, the negative ions are neutral.
Overall: Neutral solution.
NaF
1) Na+ ion
Since this is a Group IA metal ion, it is neutral.
2) F- ion
Fluoride ion is the conjugate base of the weak acid HF. Therefore, F- ion is not neutral but rather is a weak base. The conjugate base of a weak monoprotic acid will be a weak base.
Overall: Basic solution.
AlCl3
1) Al3+ ion
Most metal ions actually form acidic solutions in water. The Group IA metal ions are essentially neutral, and the Group IIA metal ions are nearly so and are usually taken to be neutral also. The transition metal ions, such as Cu2+, Fe3+, Zn2+, etc., give acidic solutions, as do small metal ions with high charges, like Al3+. A water molecule that is "complexed" to the metal ion loses H+ ion more easily than an non-complexed water molecule. I believe that six water molecules can complex with an Al3+ ion (it is not necessary to know the exact number of water molecules):
[Al(H2O)6]3+(aq) 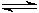 [Al(H2O)5(OH)]2+(aq) + H+(aq)
[Al(H2O)5(OH)]2+(aq) + H+(aq)
Thus the Al3+ ion causes a small increase in [H+].
2) Cl- ion
Since chloride ion is the conjugate base of the strong acid HCl, it is neutral.
Overall: Acidic solution.
Hope this helps!
Steve
 Free Forum Hosting
Free Forum Hosting