|
|
 |
Reply
 | | From:   Guy_SoCa (Original Message) Guy_SoCa (Original Message) | Sent: 3/23/2008 3:14 AM |
Regarding the energy states of the electrons in a Jablonski Diagram, what does the Singlet or Triplet state of electrons mean?
Also, is it possible for electrons to go through ISC (Intersystem crossing) from Triplet state to Singlet state?
Thanx in advanced! : ) |
|
 First First
 Previous
2-8 of 8
Next Previous
2-8 of 8
Next Last
Last
|
 |
Reply
 | | From:   ·Steve· ·Steve· | Sent: 3/23/2008 4:59 AM |
| 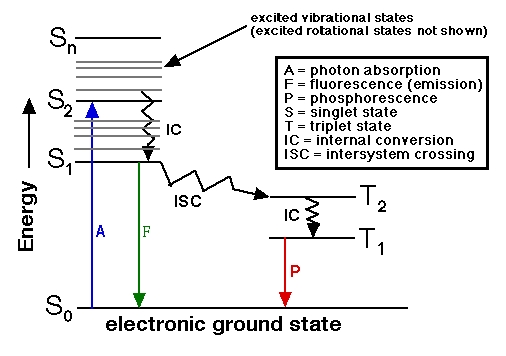 The terms "singlet" and "triplet" are spectroscopist's terms. In a magnetic field, a singlet molecule, or one that has all of its electrons paired in the same orbitals, ↑↓, or in a singlet excited state paired but in different orbitals, �? �?) exhibits only a single energy state, while a triplet molecule (a diradical having two unpaired electrons, �? �?/U> in different orbitals) exhibits three energy states corresponding to the possible orientations of the electron spins with respect to the magnetic field (both spins "up", both spins "down", or one spin "up" and the other "down"). A simple illustration using up and down arrows as electrons is shown at http://teaching.shu.ac.uk/hwb/chemistry/tutorials/molspec/lumin1.htm.
From the standpoint of total spin, if the total spin is zero (when all electrons are paired +½ and –�?adding up to zero) this is a singlet state. If you have two unpaired electrons in the molecule, +½ and +½, the total spin is +1. The spin multiplicity is given by 2S + 1. If the total spin is zero (S = 0) then the spin multiplicity 2S + 1 = 1 (a singlet state). If the total spin is 1 (S = 1) then 2S + 1 = 3 (the triplet state). Allowed electron spin transitions in atoms and molecules obey ΔS = ±1.
>> is it possible for electrons to go through ISC (Intersystem crossing) from Triplet state to Singlet state? <<
"Although intersystem crossing has been identified primarily with transitions from the lowest excited singlet state of a molecule to an even lower-lying triplet state, triplet to singlet intersystem crossing also may occur. Well-known examples of reverse (triplet to singlet) intersystem crossing include E- and P-type delayed fluorescence."
Steve
|
|
Reply
 | |
When referring to the Jablonski Diagram, which transition states are allowed and which ones are forbidden??
I could have misunderstood my teacher, and it totally contradicts the Luminol lab performed, but she said, if I heard correctly "once you go to the Triplet state, you cannot go back to the Singlet state because it would have to reverse the spin of the electrons, which is quantamechanically impossible"
but again, I could have totally misunderstood what she said.. |
|
Reply
 | | From:   ·Steve· ·Steve· | Sent: 3/24/2008 9:27 AM |
If the transition results strictly from the absorption or emission of a photon, then the allowed transitions must retain the same spin multiplicity. That is, singlet-singlet and triplet-triplet transitions would be allowed (ΔS = 0) but singlet-triplet or triplet-singlet transitions would be forbidden. These "forbidden" transitions still occur, though much more slowly, by other, non-radiative means.
Earlier I should have said ΔS = 0 for the allowed transitions. In a magnetic field, there are 2S + 1 spin energy levels (S = total spin) and allowed spin transitions obey ΔS = ±1, but this is not the case here.
I gather that it is possible for triplet-singlet transitions to occur (as noted in the reference in my previous message) but I do not know about the "reverse the spin of the electrons" problem that your teacher was referring to. Your teacher may have been thinking about the selection rules, which say that absorption or emission of a photon alone does not affect electron spin.
One problem I see it that, once intersystem crossing from and excited state singlet to excited state triplet occurs, relaxation by "internal conversion" to a lower triplet state (allowed = fast) occurs very rapidly compared to the intersystem crossing rate (forbidden = slow). Because of this, the molecule would have to go "uphill" in energy to go from the lower excited triplet state back up to a more excited triplet state that is closer to the same energy level as the excited singlet state so that intersystem crossing would be favorable.
Complicated subject!
Steve
|
|
Reply
 | | From:   ·Steve· ·Steve· | Sent: 3/24/2008 7:27 PM |
In luminol, an excited triplet dianion forms first, followed by intersystem crossing to an excited singlet state (forbidden, no emission of light yet). Then the excited singlet state goes to the singlet ground state (allowed) with emission of light.
The simple Jablonski diagrams imply that phosphorescence happens in one step when the molecule goes from an excited triplet state to the ground singlet state, but this is actually two processes: 1) change in spin multiplicity from triplet to singlet, and 2) relaxation from excited singlet to ground singlet. Some of the links below indicate these steps are occurring during the luminol phosphorescence, but the Jablonski diagram such as the one I put here earlier does not show these two steps in going from the triplet excited state to the singlet ground state. Where phosphorescence is concerned, it is often stated that this process, being forbidden, is not very probable (compared to fluorescence where the spin state does not change) but still occurs with emission of light without undergoing intersystem crossing to an excited singlet state before going to the ground singlet state. There are lots of sites dealing with research about the mechanism of phosphorescence, which I do not yet understand very well!
Steve
|
|
Reply
 | | From:   ·Steve· ·Steve· | Sent: 3/24/2008 11:16 PM |
And I forgot to mention in connection with the luminol phosphorescence, your teacher may be right and the diagram in the Wikipedia article and elsewhere may be wrong. Wiki indicates, T1 –�?gt; S1 –�?gt; So + light (Intersystem crossing, then fluorescence.) (Two steps.) But typical Jablonski diagram would indicate, T1 –�?gt; S0 + light (No intersystem crossing, just all at once with phosphorescence.) (One step or two steps?) I think your teacher was saying that the the Wiki pathway is wrong because it would not go from T1 to S1 first, as in the first pathway, just straight to S0 according to the second pathway. But I would like to know what's going on between T1 and S0 in the second pathway.  |
|
Reply
 | |
Thanx a lot!
You're the best! |
|
Reply
 | | From:   ·Steve· ·Steve· | Sent: 4/5/2008 5:05 AM |
Hey thanks, this is a topic that I need to go back and study again, been too long! Steve |
|
 First First
 Previous
2-8 of 8
Next Previous
2-8 of 8
Next Last
Last
|
|
|
 Free Forum Hosting
Free Forum Hosting